valence electrons in aluminium|Determine valence electrons using the periodic table : iloilo Chemistry of Aluminum (Z=13) Page ID. Aluminum (also called Aluminium) is the third most abundant element in the earth's crust. It is commonly used . Bethesda Game Studios welcome you to Fallout 76. Twenty-five years after the bombs fall, you and your fellow Vault Dwellers emerge into post-nuclear America. Explore a vast wasteland in this open-world multiplayer addition to the Fallout story.
valence electrons in aluminium,Mar 23, 2023 Here is a table of element valences. Remember that an element's electron .
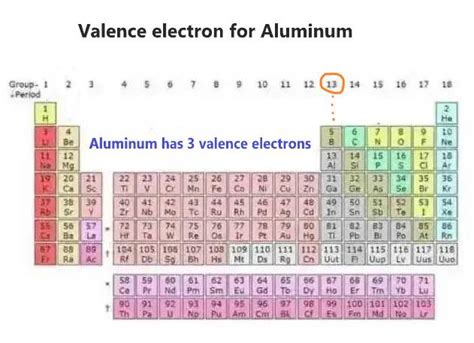
There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum .
The atomic weight of the aluminum is 26.9815. Flerovium Valence Electrons. Moscovium Valence Electrons. Livermorium Valence Electrons. . Chemistry of Aluminum (Z=13) Page ID. Aluminum (also called Aluminium) is the third most abundant element in the earth's crust. It is commonly used . Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . A Bohr model of a chlorine atom . An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell? . To find the valence electrons for aluminum (Al), you need to look at its electron configuration. Aluminum has an atomic number of 13, which means it has 13 . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Determine valence electrons using the periodic table A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. . (since metals are located on the left side of the periodic table and do not have many electrons in their valence shells). The theory must also account for all of a metal's unique chemical and physical properties.Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
Abstract. Aluminum is considered to approach an “ideal” metal or free electron gas. The valence electrons move freely, as if unaffected by the presence of the metal ions. Therefore, the electron redistribution due to chemical bonding is subtle and has proven extremely difficult to determine. Experimental measurements and ab initio .
Aluminum has 3 valence electrons because there are 3 electrons present in the outermost shell of the Aluminum (Al) atom. Now let’s see how you can easily find the valence electrons of an Aluminum atom (Al). If you don’t want to read the texts, then you can also watch this video. There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum.The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Aluminium is [Ne] 3s2 3p1.

The electron configuration of aluminum was 1 s 2 2 s 2 2p 6 3 s 2 3p 1 34. We set 1 s 2, 2 s 2 + 2p 6, and 3 s 2 + 3p 1 valence electron shells. The local axis for aluminum atom were parallel to .
Le numéro atomique de l’aluminium est 13 selon le tableau périodique. Cela signifie qu’un atome d’aluminium (Al) contient un nombre total de treize électrons. La valence est une caractéristique numérique de la capacité des atomes d’un élément donné à se lier avec d’autres atomes.

Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3 energy level which is the highest occupied energy level for magnesium. This corresponds to the 2+ 2 + charge formed .valence electrons in aluminium Determine valence electrons using the periodic table Aluminium has three extra electrons that fluorine atoms can easily use. Since aluminium has three valence electrons, three fluorine atoms can bond. AlF 3 is the chemical formula. Aluminium trichloride. Chlorine (Cl) can also form a bond with aluminium (Al). Aluminium has three extra electrons that can easily be used by chlorine atoms to bond. Boron and aluminum, for example, they can form stable molecules where the boron or the aluminum only have six valence electrons, not eight. And there are exceptions in the .
Answer: Aluminum has 3 valence electrons. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Aluminum (Al), with an atomic number of 13, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1. In this configuration, the outermost shell is the third shell (3s 2 3p 1 ), which contains 3 .valence electrons in aluminium As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871.
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .
And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system .
The shorthand electron configuration for Aluminum is [Ne] 3s 2 3p 1. The electron configuration for the Aluminum ion (Al 3+ ) is 1s 2 2s 2 2p 6. The number of valence electrons available for the Aluminum atom is 3. Aluminum is situated in Group 13th or 3A and has an atomic number of 13. Just by looking at the electron configuration, you see that there are three valence electrons. 3 electrons Aluminum is the third element in the third row of the periodic table. The outermost orbitals to be filled are 3s and 3p. The 3s orbital fills before the 3p orbitals. So, the electron configuration is [Ne]3s^2 3p^1.
valence electrons in aluminium|Determine valence electrons using the periodic table
PH0 · Valences of the Elements Chemistry Table
PH1 · Valence Electrons Chart for All Elements
PH2 · How to Find the Valence Electrons for Aluminum (Al)?
PH3 · How to Find Valence Electrons for Aluminum (Al)
PH4 · Determine valence electrons using the periodic table
PH5 · Chemistry of Aluminum (Z=13)
PH6 · Aluminum Valence Electrons
PH7 · 3.1: Valence Electrons
PH8 · 10.6: Valence Electrons